ECDOC_100393581.pdf
PHAG_Cons. EU DoC_MDR_Class I
The non-woven swab with gauze-like structure
Non-woven swab with gauze-like structure, made of 70 % viscose and 30 % polyester fibres; free of binding agents and optical brighteners; highly absorbent, soft and permeable to air; available in different plies, sterile and unsterile versions.
Sterile, sealed in packs of 5, 4-ply
non-sterile, 4-ply
| Product Information | Article Number | Content | EAN | ||||
| Sterile, sealed in packs of 5, 4-ply | |||||||
| 5x5 cm |
|
1 Folding box of 40x5 pieces 1 Box of 24 Folding boxes | 4049500265201 4049500658539 | ||||
| 7,5x7,5 cm |
|
1 Folding box of 40x5 pieces 1 Box of 24 Folding boxes | 4049500265218 4049500658553 | ||||
| 10x10 cm |
|
1 Folding box of 40x5 pieces 1 Box of 12 Folding boxes | 4049500265225 4049500658577 | ||||
| non-sterile, 4-ply | |||||||
| 10x10 cm |
|
1 Bag of 100 pieces 1 Box of 12 Bags | 4049500306355 4049500913775 | ||||
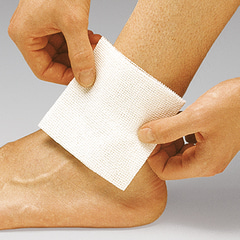
For general wound treatment; as swabs and dressings during interventions in the outpatient department and on the ward; Medicomp Drain is especially designed for use around drains, in tracheotomies and extensions and even as protection in the case of application of cannulae and probes.