ECDOC_100201778.pdf
DoC_class 1s_PHAG
The Foliodrape® standard sets are intended to prevent the passage of pathogens between non-sterile and sterile areas.
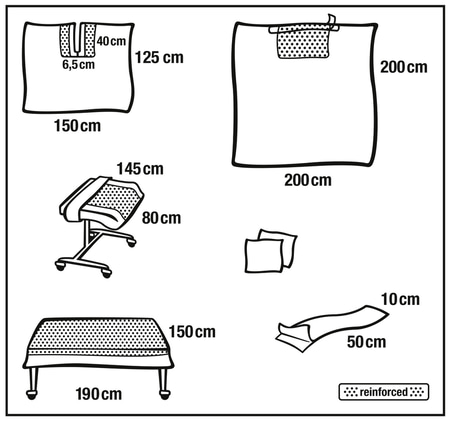
Foliodrape® ENT / Maxillofacial sets sterile are single-use products for short-term use and consists of a variety of products like patient drapes, equipment covers, fixation and collection accessories, commodity products (e.g. towels); included into sterile packages.The Foliodrape® standard sets are intended to prevent the passage of pathogens between non-sterile and sterile areas. The Polyethylene-film or different layers of hydrophilic nonwoven material laminated with Polyethylene-film or different layers of hydrophobic nonwoven material (SMS) act together as a fluid and bacteria barrier and minimize the transmission of micro-organisms. These products are placed onto the market sterile and are in Medical Device Class I.
Sterile, Individually wrapped, Brown dispenser to minimize the impact on the environment
| Product Information | Article Number | Content | EAN | ||
| Sterile, Individually wrapped, Brown dispenser to minimize the impact on the environment | |||||
|
1 Pack of 8 pieces | 4052199510187 | |||

The Foliodrape® ENT / Maxillofacial sets are used in ear, nose and throat (Otorhinolaryngology or ENT); oral; and maxillofacial surgery. Foliodrape® products are for short term use* and prevention of infection on humans in a clinical environment. Depending on the procedure for which they are used, the Foliodrape® products usage time will vary from few minutes to several hours of continuous usage.